The ICHOM Set of Patient-Centered Outcome Measures for Colorectal cancer is the result of hard work by a group of leading physicians, measurement experts and patients. It is our recommendation of the outcomes that matter most to persons with Colorectal cancer. We urge all providers around the world to start measuring these outcomes to better understand how to improve the lives of their patients.
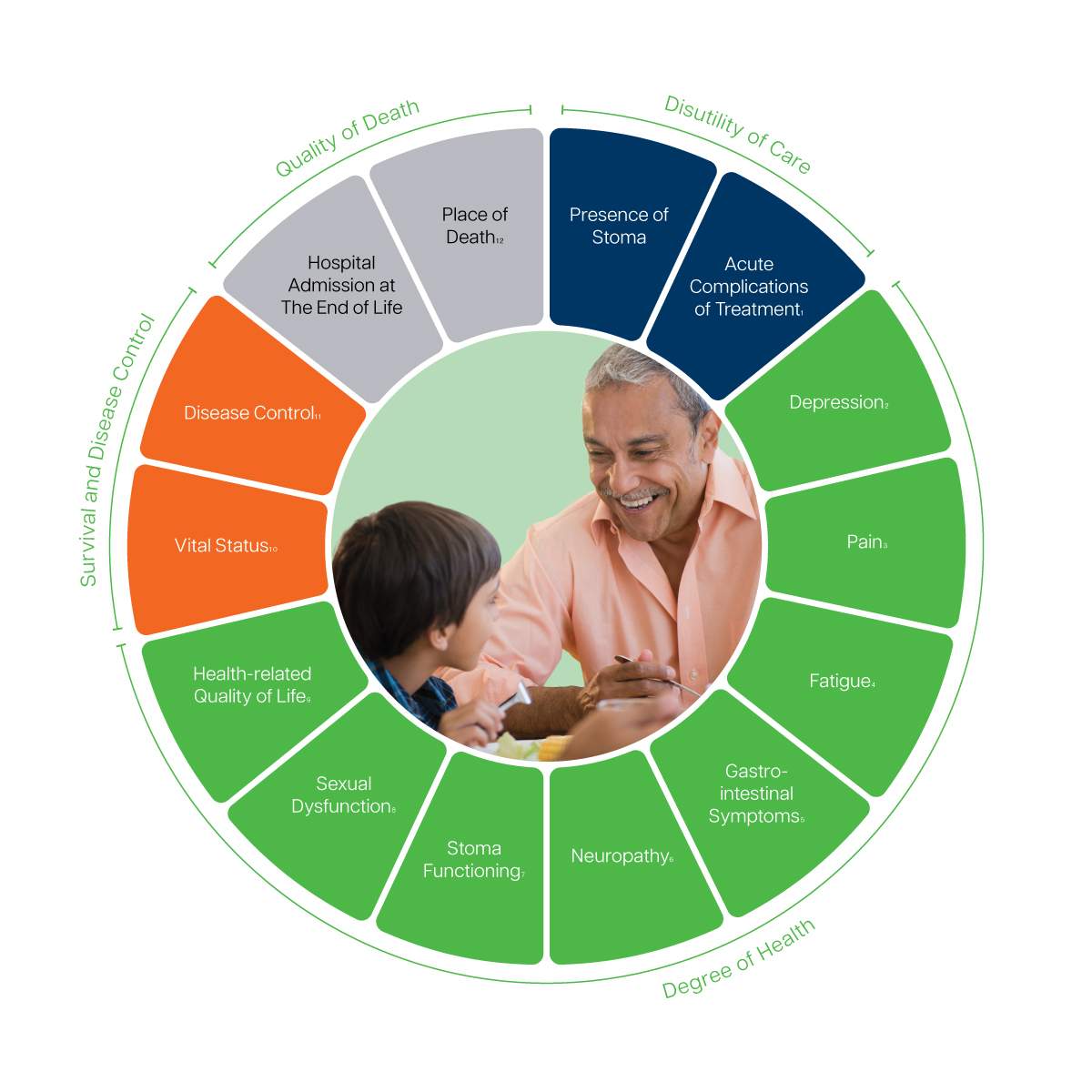
1 Complications will be recorded based on the type of therapy needed or action required to correct the complication as described in the Clavien-Dindo Classification and CTCAE v4.0.l
2,3,4 Recommeded to track via the EORTC Quality of Life Questionnaire – Core Questionnaire (EORTC QLQ-C30).
5 Includes bowel functioning, fecal leakage, stool frequency, diarrhea and dietary issues. Recommeded to track via the dietary subscale of the Memorial Sloan-Kettering Cancer Center (MSKCC) Bowel Function. Recommeded to track via the EORTC Quality of Life Questionnaire – Colorectal Cancer Module (EORTC QLQ-CR29).
6 Recommended to track via a single item from the EORTC Quality of Life Questionnaire – Liver Metastases Colorectal Module (EORTC QLQ-LMC21).
7 Recommended to track via the EORTC Quality of Life Questionnaire – Colorectal Cancer Module (EORTC QLQ-CR29).
8 Includes erectile dysfunction and vaginal symptoms. Recommended to track via the EORTC Quality of Life Questionnaire – Colorectal Cancer Module (EORTC QLQ-CR29).
9 Includes physical, emotional and social functioning and mobility and overall well-being. Recommended to track via the EORTC Quality of Life Questionnaire – Core Questionnaire (EORTC QLQ-C30).
10 Includes overall and cause-specific survival.
11 Includes pathologic complete response, margin status and recurrence and progression free survival.
12 Includes place of death and preference for place of death according to the patient.
Team that developed this set
AUSTRALIA
Donna Bauer* | Bowel Cancer Australia
Craig Lynch | Peter MacCallum Cancer Center
John ZalcBerg | Monash University
BELGIUM
Eric van Cutsem | Leuven Cancer Institute
GERMANY
Corinna Langelotz | Universitätsmedizin Berlin-Charité
NETHERLANDS
Rob Tollenaar | Leiden University Medical Center
Cornelis van de Velde | Leiden University Medical Center
Eino van Duyn | Medisch Spectrum Twente
MALAYSIA
Muhammad Radzi Abu Hassan | Hospital Sultanah Bahiyah
SINGAPORE
Joanne Ngeow | National Cancer Centre Singapore
Jessica Zerillo | Beth Israel Deaconess Medical Center
SPAIN
Josep Borras | University of Barcelona
TAIWAN
Skye Hung-Chung Chen | Koo Foundation Sun Yat-Sen Cancer Center
UNITED KINGDOM
Michael Fenn* | AOL community
UNITED STATES
Ann Berger | University of Nebraska Medical Center
Giles Boland | Harvard Medical School
Robert Cima | Robert Cima
Sam Finlayson | University of Utah
John Lloyd* | Colon Cancer Alliance
Harvey Mamon | Dana Farber Cancer Institute
Pamela McAllister* | Fight Colorectal Cancer
Bruce Minsky | MD Anderson Cancer Center
Kim Ryan* | Cancer Support Community
Veena Shankaran | University of Washington Medical Center
Melissa Upton | University of Washington Medical Center
*Patient representative
Are you implementing ICHOM Sets?
If your are implementing ICHOM Sets, please help us understand more about your journey by filling in our Implementation Survey. Click on the link below to complete:
Implementation Map
We would like to add you to our Implementation Map if you are implementing or have implemented ICHOM Sets. Please click on the button below for more information.







































